Valsartan Recall Information for Patients and Prescribers
UPDATE: On August 18, 2018, as a precautionary measure, Teva Canada expanded its voluntary recall to include eight additional lots of valsartan products in Canada (Health Canada – Information Update).
Several drugs containing the ingredient valsartan are being recalled by their manufacturers. An impurity, N-nitrosodimethylamine (NDMA), was found in the valsartan used in these products. The valsartan was supplied by Zhejiang Huahai Pharmaceuticals. NDMA is a potential human carcinogen, which means that it could cause cancer with long-term exposure. Five companies have affected products, which are being recalled.
Drugs containing valsartan are used to treat patients with high blood pressure to help prevent heart attacks and stroke. These drugs are also used in patients who have had heart failure or a recent heart attack.
Canadians who are taking any medication containing valsartan are advised to:
- Speak to their pharmacist who can tell them if their medicine is being recalled.
- Contact their health care practitioner as soon as possible to discuss their treatment options.
- Continue taking their medication if it contains valsartan unless they are told to stop by their health care professional.
For additional information, including a list of all valsartan products affected see: Recall Advisory - Health Canada
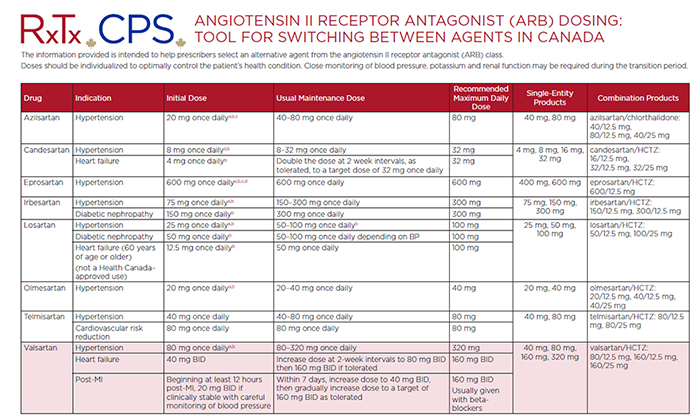
Therapeutic Options Tool
CPhA has developed a tool to help prescribers select an alternative agent from the angiotensin II receptor antagonist (ARB) class. The information comes from our existing Angiotensin II Receptor Antagonist (ARB) CPS Monographs and Drug Tables located in RxTx. Doses should be individualized to optimally control the patient’s health condition. Close monitoring of blood pressure, potassium and renal function may be required during the transition period.